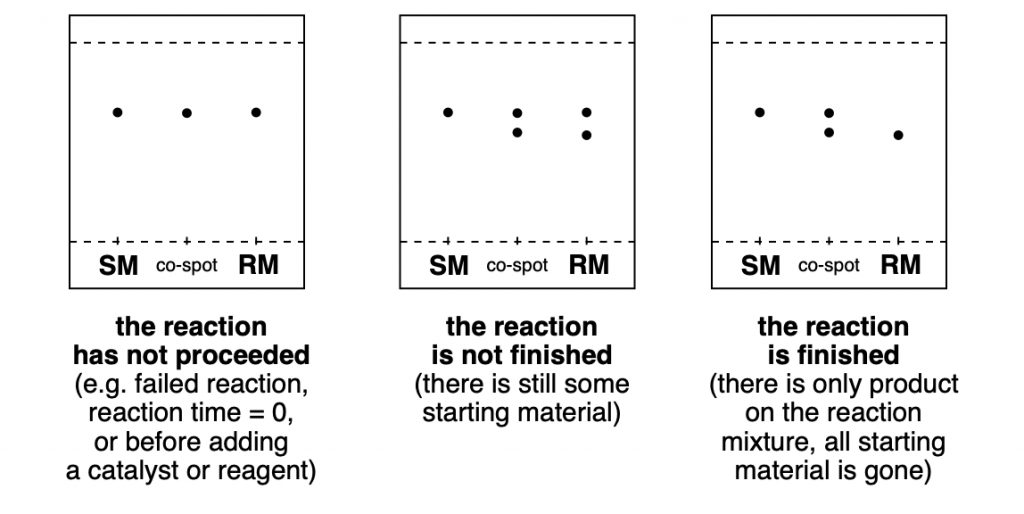
Chromatography Which Is More Polar . It is also used to determine the. In this section are discussed the details of the separation, and expand upon. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. the larger the charge difference, the more polar a molecule is. chromatography is a method in which a mixture of compounds is separated into its individual components. sample purity is achieved using chromatography—the process of separating components of a mixture. Tlc is an excellent analytical tool for separating mixtures in a sample. So, how long do you spend performing. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. You will find that as you increase the polarity of the solvent,.
from mungfali.com
the larger the charge difference, the more polar a molecule is. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. In this section are discussed the details of the separation, and expand upon. chromatography is a method in which a mixture of compounds is separated into its individual components. So, how long do you spend performing. You will find that as you increase the polarity of the solvent,. It is also used to determine the. sample purity is achieved using chromatography—the process of separating components of a mixture. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. Tlc is an excellent analytical tool for separating mixtures in a sample.
Thin Layer Chromatography Polarity
Chromatography Which Is More Polar It is also used to determine the. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. sample purity is achieved using chromatography—the process of separating components of a mixture. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. the larger the charge difference, the more polar a molecule is. In this section are discussed the details of the separation, and expand upon. You will find that as you increase the polarity of the solvent,. chromatography is a method in which a mixture of compounds is separated into its individual components. It is also used to determine the. So, how long do you spend performing. Tlc is an excellent analytical tool for separating mixtures in a sample.
From www.youtube.com
Which Bond is More Polar Practice Problems, Examples, Shortcut Chromatography Which Is More Polar You will find that as you increase the polarity of the solvent,. the larger the charge difference, the more polar a molecule is. chromatography is a method in which a mixture of compounds is separated into its individual components. In this section are discussed the details of the separation, and expand upon. It is also used to determine. Chromatography Which Is More Polar.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Chromatography Which Is More Polar in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. So, how long do you spend performing. It is also used to determine the. sample purity is achieved using chromatography—the process of separating components of a mixture. thin layer chromatography (tlc) is. Chromatography Which Is More Polar.
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide Chromatography Which Is More Polar thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. chromatography is a method in which a mixture of compounds is separated into its individual components. It is also used. Chromatography Which Is More Polar.
From slideplayer.com
Chemistry Work on “Chem Phys Properties” Today ppt download Chromatography Which Is More Polar So, how long do you spend performing. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. You will find that as you increase the polarity of the solvent,. sample. Chromatography Which Is More Polar.
From mungfali.com
Thin Layer Chromatography Polarity Chromatography Which Is More Polar Tlc is an excellent analytical tool for separating mixtures in a sample. the larger the charge difference, the more polar a molecule is. So, how long do you spend performing. chromatography is a method in which a mixture of compounds is separated into its individual components. It is also used to determine the. in normal phase chromatography,. Chromatography Which Is More Polar.
From lab-training.com
Gas Chromatography Principles, Types and Working Chromatography Which Is More Polar You will find that as you increase the polarity of the solvent,. It is also used to determine the. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. sample purity is achieved using chromatography—the process of separating components of a mixture. . Chromatography Which Is More Polar.
From dxortyzmw.blob.core.windows.net
What Is Polarity In Chromatography at Sarah Ramos blog Chromatography Which Is More Polar You will find that as you increase the polarity of the solvent,. chromatography is a method in which a mixture of compounds is separated into its individual components. It is also used to determine the. Tlc is an excellent analytical tool for separating mixtures in a sample. thin layer chromatography (tlc) is an extremely useful technique for monitoring. Chromatography Which Is More Polar.
From solvedlib.com
Nwhich Chromatography stationary phase is more polar … SolvedLib Chromatography Which Is More Polar In this section are discussed the details of the separation, and expand upon. You will find that as you increase the polarity of the solvent,. chromatography is a method in which a mixture of compounds is separated into its individual components. Tlc is an excellent analytical tool for separating mixtures in a sample. thin layer chromatography (tlc) is. Chromatography Which Is More Polar.
From stock-picture.com
thin layer chromatography image Big Image Stock Chromatography Which Is More Polar So, how long do you spend performing. sample purity is achieved using chromatography—the process of separating components of a mixture. It is also used to determine the. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. chromatography is a method in which a mixture of compounds is separated into its individual components. You will. Chromatography Which Is More Polar.
From www.youtube.com
Paper Chromatography Intro & Theory YouTube Chromatography Which Is More Polar in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. You will find that as you increase the polarity of the solvent,. In this section are discussed the details of the separation, and expand upon. chromatography is a method in which a mixture. Chromatography Which Is More Polar.
From vdocuments.mx
General · PDF fileGeneral Chromatography Solvent Polarity Chart Chromatography Which Is More Polar thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. sample purity is achieved using chromatography—the process of separating components of a mixture. You will find that as you increase the polarity of the solvent,. Tlc is an excellent analytical tool for separating mixtures in a sample. in normal phase chromatography, the stationary phase is. Chromatography Which Is More Polar.
From www.slideserve.com
PPT classification of chromatography PowerPoint Presentation, free Chromatography Which Is More Polar chromatography is a method in which a mixture of compounds is separated into its individual components. It is also used to determine the. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. Tlc is an excellent analytical tool for separating mixtures in a sample. the larger the charge difference, the more polar a molecule. Chromatography Which Is More Polar.
From www.slideserve.com
PPT Chromatography PowerPoint Presentation, free download ID542840 Chromatography Which Is More Polar sample purity is achieved using chromatography—the process of separating components of a mixture. You will find that as you increase the polarity of the solvent,. Tlc is an excellent analytical tool for separating mixtures in a sample. It is also used to determine the. the larger the charge difference, the more polar a molecule is. chromatography is. Chromatography Which Is More Polar.
From chem.libretexts.org
12.2 General Theory of Column Chromatography Chemistry LibreTexts Chromatography Which Is More Polar chromatography is a method in which a mixture of compounds is separated into its individual components. the larger the charge difference, the more polar a molecule is. So, how long do you spend performing. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary. Chromatography Which Is More Polar.
From www.slideshare.net
Chromatography Chromatography Which Is More Polar So, how long do you spend performing. chromatography is a method in which a mixture of compounds is separated into its individual components. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. Tlc is an excellent analytical tool for separating mixtures in a sample. sample purity is achieved using chromatography—the process of separating components. Chromatography Which Is More Polar.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID298459 Chromatography Which Is More Polar Tlc is an excellent analytical tool for separating mixtures in a sample. In this section are discussed the details of the separation, and expand upon. sample purity is achieved using chromatography—the process of separating components of a mixture. You will find that as you increase the polarity of the solvent,. chromatography is a method in which a mixture. Chromatography Which Is More Polar.
From www.youtube.com
Molecular Polarity used in Chromatography YouTube Chromatography Which Is More Polar sample purity is achieved using chromatography—the process of separating components of a mixture. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. In this section are discussed the details of the separation, and expand upon. You will find that as you increase. Chromatography Which Is More Polar.
From dxortyzmw.blob.core.windows.net
What Is Polarity In Chromatography at Sarah Ramos blog Chromatography Which Is More Polar You will find that as you increase the polarity of the solvent,. in normal phase chromatography, the stationary phase is polar, and so the more polar solutes being separated will adhere more to the stationary adsorbent phase. thin layer chromatography (tlc) is an extremely useful technique for monitoring reactions. It is also used to determine the. chromatography. Chromatography Which Is More Polar.